Medical Device Failures and Patient Injury Caused by Packaging Errors
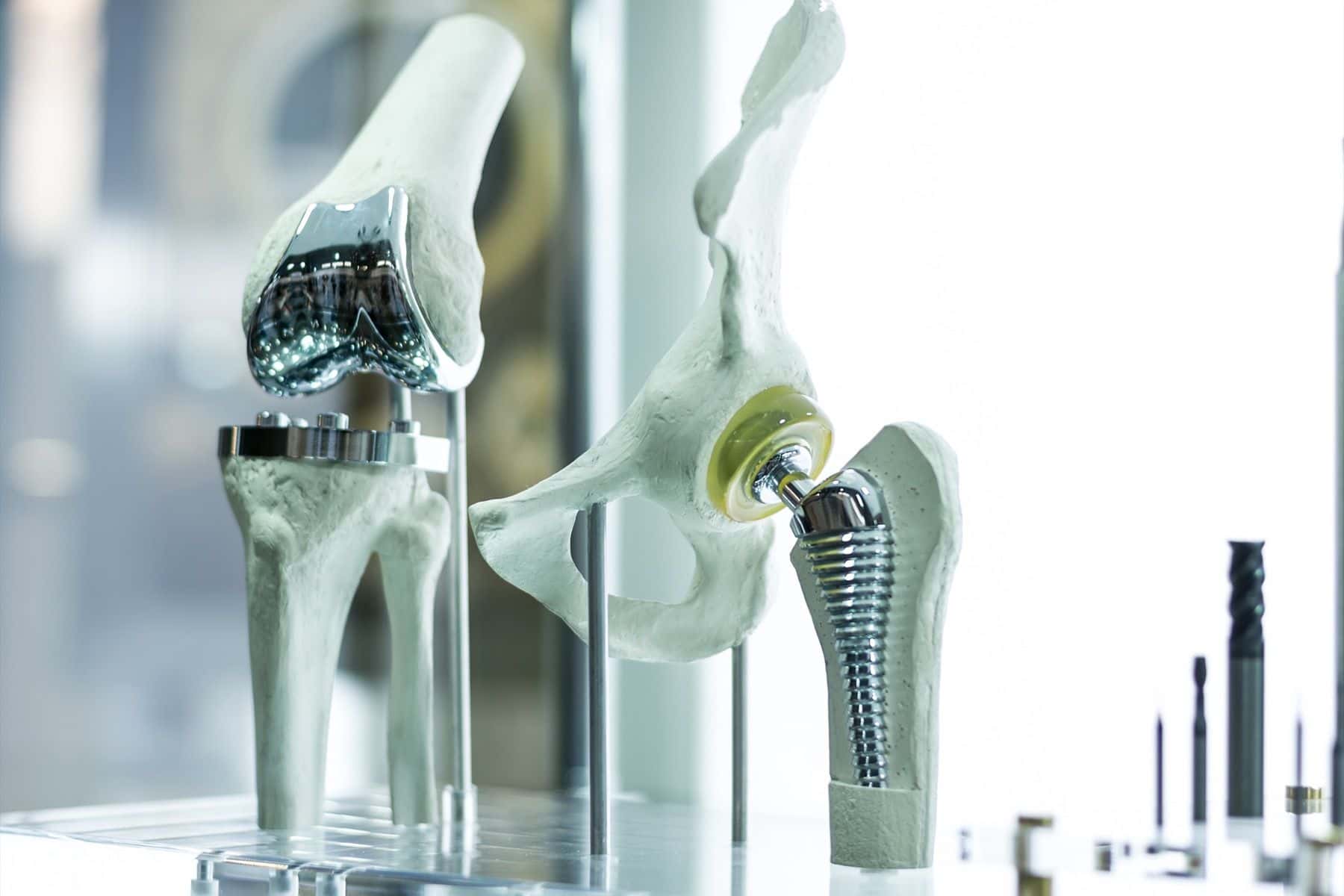
This case study explores a situation where an adult patient sustained injuries due to a defective tibial insert, allegedly caused by improper packaging leading to oxidation.
Updated on
Case Overview
In this case study, we examine medical complications surrounding an adult patient who sustained injuries due to a defective tibial insert. It is alleged that this defect was attributed to improper packaging, which resulted in the oxidation of the device’s polyethylene component.
The case seeks expert opinion on the importance of following correct packaging procedures for medical devices, particularly when dealing with oxidative materials like polyethylene.
Questions to the Medical Device expert and their responses
Please provide examples of your professional background in medical device packaging.
I have over 20 years’ experience in the medical device industry, focusing on regulatory compliance and quality assurance aspects. My work involves adhering to packaging and other types of standards during the manufacture and packaging of orthopedic devices.
What is the proper way to package a medical device made of polyethylene to prevent oxidation?
To ensure optimal oxidation protection, it is crucial to follow FDA-recognized ASTM packaging standards, including ISO 11607-1. Depending on the specific materials used in the device, other standards may also be applicable. If a device is not packaged correctly, it can lead to issues like oxidation, which can compromise its functionality and safety.
Additionally, if the tibial insert was oxidized due to faulty packaging, the device may not have been properly sterilized either.
About the expert
This expert boasts over two decades of experience in regulatory affairs, including FDA and drug safety. Their extensive educational background includes a BA in Biochemical Sciences from a prestigious Ivy League university and both an MBA and PhD in Molecular and Cell Biology from a well-known institution. Currently serving as a professor of global regulatory affairs at a Massachusetts university. This expert also holds significant roles in bioscience regulatory consulting and has held high-ranking positions at several medical technology companies, making them relevant to the case.

E-307061
Specialties:
About the author
Subscribe to our newsletter
Join our newsletter to stay up to date on legal news, insights and product updates from Expert Institute.
Sign up nowFind an expert witness near you
What State is your case in?
Subscribe to our newsletter
Join our newsletter to stay up to date on legal news, insights and product updates from Expert Institute.