Research Guides
Cutting-edge legal research powered by AI
Access medico-legal research papers prepared by our researchers and physicians - driven by artificial intelligence.
Have an iQ account?
Log in to get started
Get the facts. Analyze your options.
Accelerate your case research with AI
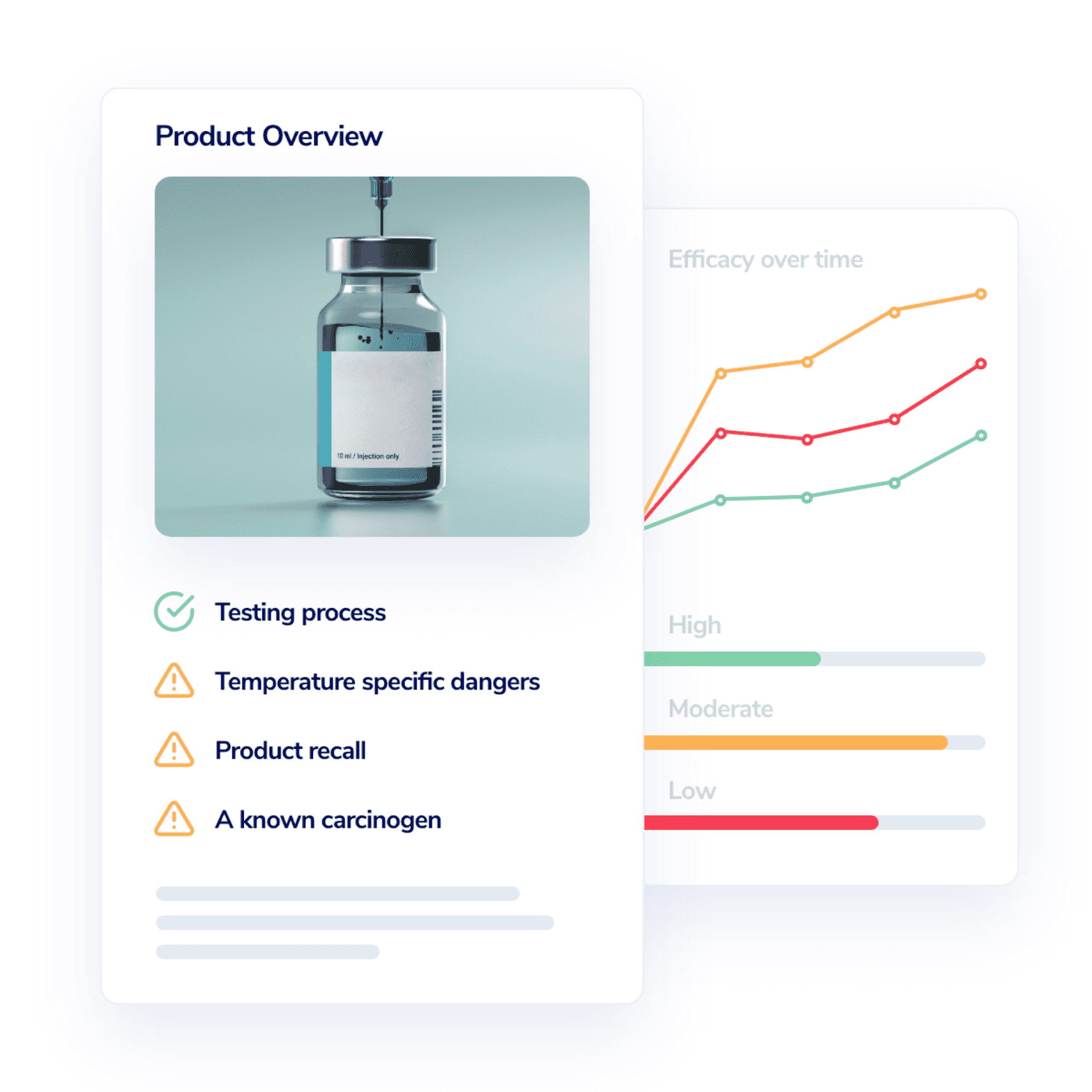
Accelerate your case research with AI
Artificial intelligence speeds up the literature review process, enabling our researchers to quickly find answers, and provide a comprehensive understanding of the science behind any injury, condition, device, or procedure.
Accelerate your researchStreamline your case theory and solidify causation
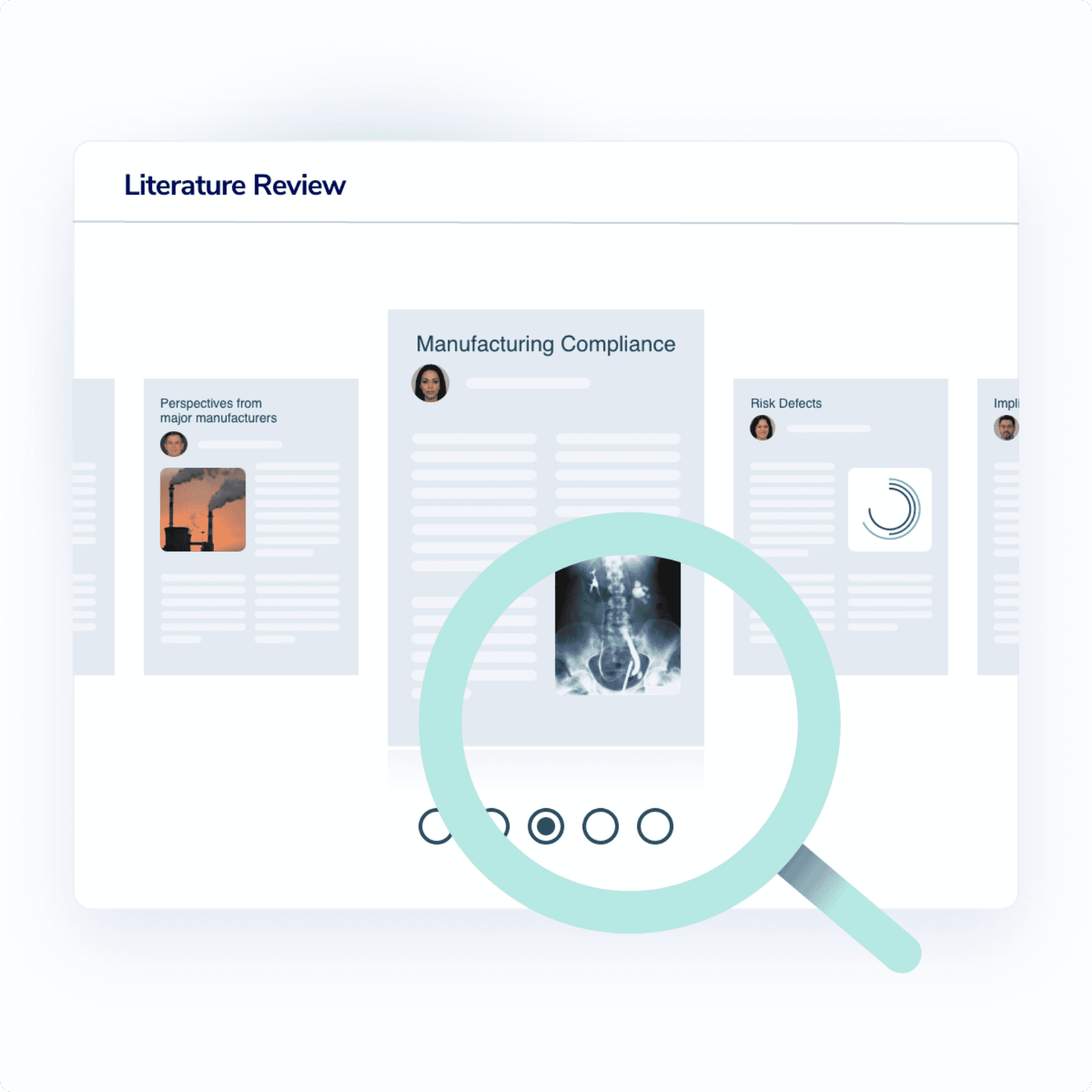
Streamline your case theory and solidify causation
Explore litigation research that strengthens your legal theory, and identifies existing counterarguments to ensure you raise the most important questions at deposition or trial.
Strengthen your theoryAccess the best research

Access the best research
Refute an opposing expert’s opinion, construct complaint documents, craft a compelling report, clarify standards of care, and more.
Win more casesLegal research to help you navigate every case
Writing a complaint?
We’ll arm you with a crystal clear medical perspective and sound legal theory, so that you can craft a decisive complaint.
Need an insurance company report?
Our medical team will provide detailed documentation summarizing your client’s injury, and the necessary ongoing healthcare coverage.
Have a policy question?
Our doctors can outline the standard of care - from COVID protocols, emergency room triage timeframes, to patient safety guidelines.
Medico-legal research tailored to your case
Enhance your case research
Streamline your theory
Prepare for the win
Voted #1 Expert Witness Provider By


