Transvaginal Mesh Expert Witness: A Litigation Guide
In recent years, complaints have mounted against companies producing transvaginal mesh products and therefore the employment of the transvaginal mesh expert witness. As these cases head to trial, the specific issues and elements of litigation need to be understood. At The Expert Institute, we have extensive experience with these unique considerations, and we’ve compiled this guide to help attorneys and injured victims litigating transvaginal mesh claims.
Updated on
Medical Summary
What is Pelvic Organ Prolapse (POP)?
Pelvic organ prolapse (POP) is a medical condition in which female pelvic organs—such as the bladder, uterus, and vagina—descend from their normal positions within the pelvis. In some cases, these organs can protrude through the vaginal opening.
Prevalence and Severity
- Common Condition: Symptomatic in approximately 30% of women aged 50-89.
- Surgical Intervention: 11% require corrective surgery by age 80.
Risk Factors for POP
POP occurs frequently with normal aging in women who have:
- Had multiple vaginal deliveries
- Had a prior hysterectomy
- A high BMI, which predisposes women to the weakening of supporting ligaments and muscles in the pelvic floor.
Surgical Statistics and Future Demand
- Annual Procedures: Researchers estimate that there are up to 250,000 surgeries in the U.S. each year to correct POP.
- Projected Increase: Expected to rise by 50% as the population of older women grows.
Treatment Option: Sacrocolpopexy
Sacrocolpopexy is a surgical technique used to treat vaginal vault or uterine prolapse.
The objectives of the procedure are to:
- Reduce the extent of the prolapse.
- Restore vaginal anatomy and function.
During the procedure, the apex of the vagina and base of the uterus is lifted back up to its natural position by attaching a synthetic mesh from the top and back of the vagina to the sacral promontory.
The mesh provides the vagina with the right amount of support to keep it in the correct position.
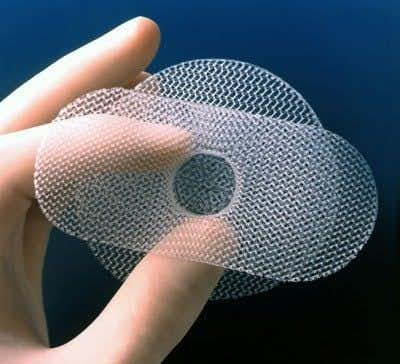
Transvaginal Mesh Implants
The use of standardized mesh kits for repair of pelvic organ prolapse has spread rapidly in recent years. It is unclear whether this approach results in better outcomes than colporrhaphy, which is the traditional surgical technique used to repair defects in the vaginal wall.
Mesh devices are implanted to provide additional support when repairing weakened or damaged tissue, and the majority of surgical mesh devices currently available for use are made from synthetic materials or animal tissue.
The absorbable mesh will degrade and lose strength over time, as it is not intended to provide long-term reinforcement to the repair site.
As the material degrades, new tissue growth is intended to provide strength to the repair. Surgical mesh can be used for urogynecologic procedures, including repair of pelvic organ prolapse and stress urinary incontinence (SUI).
Three Types of Transvaginal Mesh Design
Tension-free vaginal tape (TVT) sling
This variation of the device is principally used for the treatment of stress incontinence.
During Implantation:
The mesh is inserted through the vagina along with two small incisions in the lower abdomen. Unlike other transvaginal procedures, sutures and bone anchors are not required.
Risks of the TVT Technique:
The procedure requires the surgeon to blindly pass a needle through the narrow retropubic space, which contains highly vascular tissues and is close to the bowel and bladder. Surgical skill is crucial for success, as the area leaves little room for error.
Trans-obturator tape (TOT) sling
As a result of serious complications from TVT technique (some that hinged upon the blind needle passage method), surgeons created a novel approach called the trans-obturator tape procedure.
Risk Mitigation of the TOT Technique:
This procedure eliminates the need for a needle in the retropubic space. Instead, the needle is placed through a less vascularized area of the groin, reducing the risk of internal injuries compared to TVT.
Mini-sling
Primarily used for stress urinary incontinence (SUI), this method uses only one vaginal incision under the urethra.
Procedure Details:
This variation employs one slim 2.3mm needle designed to minimize tissue trauma and help promote less dissection. A fixed needle to sling tip connection was originally designed to prevent mesh rotation and disengagement until the slider mechanism is depressed. During disengagement, the needle retracts into the needle shaft to help deliver proper tensioning of the device.
Safety Benefits:
Some physicians find it to have a lower risk of internal injuries compared to the TVT method.
Legal Summary
FDA Approval and Early Usage
Transvaginal mesh was initially approved by the FDA in 1996, although its usage dates back to the 1950s.
- First Product: Boston Scientific's 'ProtoGen' vaginal sling, approved by the FDA in 1996, was used to treat stress urinary incontinence.
- Recall: Product recalled in January 1999 due to numerous complaints.
Expanded Approval and Increasing Complications
Despite the dangers of the device, other companies, including Johnson and Johnson, had started to market their own transvaginal meshes.
- Growing Usage: In 2002, the FDA approved transvaginal meshes for the treatment of pelvic organ prolapse.
At this point, transvaginal meshes were frequently used to treat stress urinary incontinence and, increasingly, POP.
As the frequency of their usage increased, reports of complications began to grow as well:
- 2008 Notification: The FDA issued a public health notification, stating that it had received over 1,000 complaints in three years related to transvaginal mesh products.
Some of the more common complaints included infections, pain, and bowel and bladder perforations. - 2011 Warning: The FDA issued another public health warning, stating that it had received 2,864 complaints.
They stated that “Serious injuries associated with surgical mesh for transvaginal mesh repair of pelvic organ prolapse were not rare.” - 2012 Study: The FDA told 33 manufacturers to study rates of organ damage and complications linked to vaginal mesh implants.
Looking for an expert witness? Connect with a vetted and screened expert in any discipline.
Gross Case
On February 25th, 2013, the Superior Court in New Jersey ruled that a South Dakota woman who received a transvaginal mesh was entitled to damages because of, “their defective design, manufacture, warning, and instructions.”
To provide some context, this was the first of more than 4,000 cases to go to trial against Johnson & Johnson (and their subsidiary, Ethicon), and the first of more than 1,800 such cases to go to trial in New Jersey. Overall, analysts estimate that there are over 10,000 such cases pending against manufacturers of transvaginal meshes.
In the case, Linda Gross filed suit against Ethicon and Johnson & Johnson after she had a Gynecare PROLIFT installed in July of 2006. Gross alleged that, because of the mesh, she suffered mesh erosion, scar tissue, inflammation, and neurologic compromise. Because of the harm, and over twenty-two corrective surgeries to remove most, but not all, of the device, Gross brought suit against J&J and Ethicon in November 2008 in New Jersey Superior Court.
Upon ruling in favor of the plaintiff, the court awarded $3.35 million in compensatory damages. The jurors awarded $1.1 million for pain and suffering, $180,000 for lost wages, $500,000 for future lost wages, $385,000 for past medical treatment, $1 million for future medical treatment, and $180,000 for husband’s loss of companionship. Additionally, on March 1, the jurors awarded $7.76 million in punitive damages. Linda Gross’s case is going to act as an illustrative model, with an approach that other attorneys may follow when litigating similar claims. For instance, a number of cases are being litigated against Boston Scientific Corporation, C.R. Bard Inc., and Ethicon in U.S. District Court, Southern District of West Virginia. Furthermore, according to an order issued on April 25th, the bellwether case for C.R. Bard, Inc in New Jersey is scheduled for September 23, 2013.
With numerous bellwether cases to be decided, the specifics of the Gross decision are important. In her case, Gross’s attorneys called a number of expert witnesses that helped to provide context and color to her claims. The plaintiff called an expert to project estimated economic losses, an expert on electrical engineering to measure the mesh’s pores, a psychiatrist to measure the plaintiff’s psychological trauma, and a transvaginal mesh expert witness in pain management. In addition to the medical experts selected (including a urogynecologist, a critical transvaginal mesh expert witness), Gross’s selection of relevant and quality experts helped to give the jury a better sense of the harm caused to her, further proving how the injury impacted her life. This undoubtedly led to a larger damage award and may set a precedent for punitive damage awards in similar cases.
Complications of Transvaginal Mesh
Infection
Due to the nature of the mesh placement, in addition to the materials used, transvaginal meshes present a number of infection-based risks for the patient. In some cases, lack of treatment can be life-threatening. Generally, there are three types of infections that may be caused by a transvaginal mesh: a kidney infection, a bladder or urinary tract infection, or a blood infection. Out of the three types, a blood infection (sepsis) is the most serious. Sepsis is a potentially fatal condition; once bacteria gains access to the bloodstream, it is a battle for the body to defend itself against invading microorganisms. The body is under constant attack from external sources and the inherent inflammatory response to the foreign body usually causes more systemic damage than the primary infection itself.
Vaginal Scarring
In pelvic organ prolapse, the mesh is implanted to adjust the positioning of the internal structures of the vagina. The device was originally designed to support and recreate the vaginal vault and to increase the stability of the pelvic cavity preventing future prolapse of pelvic tissue. The process of implanting the mesh, however, can actually cause extensive scar tissue to develop across multiple areas of the vagina. This can cause a number of related health issues including a predisposition to infection, incontinence issues, and other genito-urinary problems. The scarring can lead to painful sexual intercourse and require subsequent surgeries to remove excess scar tissue, which may obstruct the vaginal canal and impinge on internal pelvic organs and surrounding urinary tract tissue.
Painful Sexual Intercourse
While likely associated with the previous two serious side effects of vaginal mesh implants, painful sexual intercourse has a unique element to it. The loss of intimacy is a major psychological issue that can cause significant harm to one’s life — an issue that can be brought up in a suit. The causes are often reversible, even when long-standing, but self-perpetuating pain is a factor after the original cause has been removed and many years of psychotherapy may be needed for a full recovery. Furthermore, damages may be awarded to ones spouse (assuming the claimant is married) – further expanding monetary recovery, as the loss of intimacy will affect both individuals.
Chronic pelvic area pain
The most nonspecific complication, chronic pelvic pain, is a multifactorial syndrome, which can be linked to many patients with mesh implants. In most cases, it is actually the first sign that something is wrong after surgery. The absence of visible pathology in chronic pain syndromes often delays diagnosis since physicians lean toward a psychological cause and question the reality of the patient’s pain. Instead, it is essential to approach the complexity of chronic pain from a psychophysiological perspective, which completely recognizes the importance of the mind-body interaction.
The Transvaginal Mesh Expert Witness
Obstetrician/Gynecologist
An obstetrician and gynecologist is a physician extensively trained in the skills and professional capabilities of the medical and surgical care of the female reproductive system and associated disorders. An obstetrician/gynecologist is also involved as a primary care consultant to other physicians for the entirety of a woman’s life cycle. In particular, an obstetrician/gynecologist has expertise in the management of pregnancy, labor, puerperium, prenatal care, detection of sexually transmitted diseases, pap test screening, oncology, reconstructive surgery, as well as family planning. An obstetrician/gynecologist is the ideal medical professional needed to speak on the procedure and/or management of complications in relation to the transvaginal mesh procedure.
Urologist/Urogynecologist
A urologist is a surgeon extensively trained in the skills and professional capabilities dealing with the diseases of the male and female urinary tract, as well as the male reproductive organs. A urologist can be further trained in pediatric urology, oncology, renal transplantation, male infertility, calculi, female urology, and neurology. In particular, a urologist with fellowship training in female urology has in-depth knowledge of urinary incontinence and pelvic outlet relaxation disorders. A urologist with a fellowship in female urology, otherwise known as a urogynecologist, is the ideal medical professional to speak on the surgical procedures and/or products used for the correction of pelvic organ prolapse and stress urinary incontinence.
Biomechanical Engineer
Biomedical engineers can serve in a variety of roles, most of which involve the invention and development of medical devices such as implants, meshes, prostheses, and hardware used to help improve a patient’s health and quality of life. These engineers can draw on a strong knowledge base to speak on the processes of design control, failure modes, effects analysis, and clinically relevant design safety protocols including device performance requirements during medical device litigation reviews. Additionally, these experts can provide insight into the principles of engineering mechanics to explain the basic biological processes and mechanisms related to the structure, function, and injury threshold of body tissue, with emphasis on bone and other tissues that would be relevant to transvaginal mesh litigation.
Expert Life Care Planner
An expert life care planner is a consultant with expertise in the vocational evaluation and life planning for use in a number of medical injury cases. In particular, an expert life planner has knowledge and experience working with personal injury, medical malpractice, product liability, wrongful termination, and other medical damage related cases. An expert life care planner is the ideal professional in assessing the long-term costs and/or care specific preparation in accordance to any injuries sustained in a transvaginal mesh procedure.
About the author
Michael Morgenstern
Michael is Senior Vice President of Marketing at The Expert Institute. Michael oversees every aspect of The Expert Institute’s marketing strategy including SEO, PPC, marketing automation, email marketing, content development, analytics, and branding.
Subscribe to our newsletter
Join our newsletter to stay up to date on legal news, insights and product updates from Expert Institute.
Sign up nowA Sample Voir Dire: How To Qualify An Expert Witness
Download free white paperChallenging Opposing Experts: Advanced Research Techniques
Download free white paperCross Examining Expert Witnesses: The Ultimate Guide
Download free white paper
Subscribe to our newsletter
Join our newsletter to stay up to date on legal news, insights and product updates from Expert Institute.