Litigation Guides
Paragard IUD Lawsuits: A Guide for Attorneys
The Paragard IUD, on the market since the 1980s, is an intrauterine device offering long-term contraception. The device, however, has become the focus of an increasing number of lawsuits by consumers alleging that the IUD can lead to serious injuries. In this litigation guide, we cover the risks reported by Paragard users, the ongoing lawsuits against the IUD’s maker, and how experts will come into play as these cases proceed. For attorneys pursuing Paragard-related litigation, here’s what you need to know.
Written By
Wendy Ketner, M.D.
Medically Reviewed
What is the Paragard IUD?
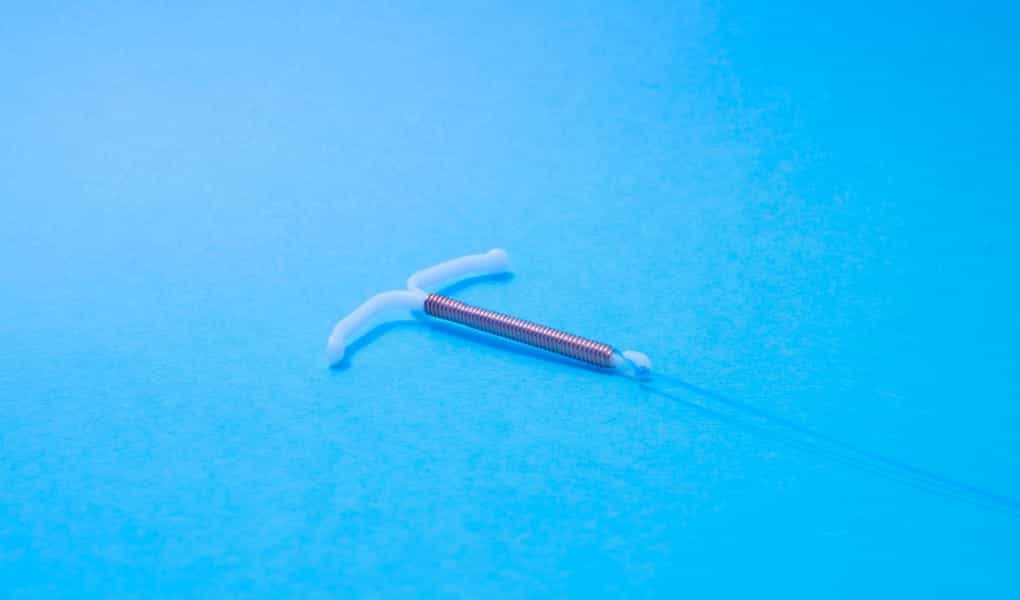
The Paragard IUD is a hormone-free, T-shaped device with an outer copper coating. Paragard has been available in the United States since 1988, after receiving FDA approval in 1984. It is currently manufactured and marketed by Cooper Companies after its acquisition from Teva Pharmaceuticals in 2017.
Paragard is commonly used to prevent pregnancy. Since the IUD begins working immediately upon insertion, it can also be used as a means of emergency contraception. Unlike Plan B pills, the weight of the patient is not a factor in its effectiveness.
Currently, five different IUDs are available in the United States. Paragard is the only one of the five that works without secreting levonorgestrel. This hormone can cause problematic symptoms in some patients. Paragard is also the oldest IUD currently on the market.
Paragard IUDs are not the first brand of IUD to face challenges in court. Medical device manufacturer Bayer faced lawsuits claiming its Mirena IUD had migrated from the uterus. These claims alleged the device damaged other organs in several patients who then needed surgery to treat these complications. Bayer eventually settled 4,600 claims for a total of $12.2 million in 2018.
Paragard Use and Common Side Effects
The Paragard IUD is typically inserted in a health care provider’s office. A thin plastic tube is used to insert the intrauterine device into the uterus through the vagina. A pair of threads hang from the IUD into the vagina to allow for later removal. The Paragard IUD is approved for use for up to ten years.
Patients may prefer Paragard to other IUD options because it does not administer hormones. This means that Paragard users do not face the potential side effects or risks of levonorgestrel use. This does not mean Paragard is side-effect-free, however. Side effects listed in the prescribing information include anemia, backache, dysmenorrhea (painful periods), unusually heavy period flow, menstrual spotting, pain and cramping, vaginitis, dyspareunia (pain with intercourse), and complete or partial expulsion.
Paragard IUD Associated Risks
Paragard is also associated with more serious risks—many of which have become the basis for ongoing litigation against IUD makers.
Breakage or Embedding of Paragard Devices
Many patients choose Paragard because its in-office, surgery-free insertion process seems to pose fewer risks than other surgical options for contraception. Many of the active lawsuits against manufacturer Teva Pharmaceuticals, however, argue that the Paragard IUD comes with risks that were never fully explained to its users.
Patients claimed that they weren’t informed that the Paragard could potentially break. This breakage poses a risk of pieces of the IUD either becoming embedded in the uterine wall or damaging other organs. This, in turn, can also lead to further health problems.
Paragard IUD’s current prescribing information states that “device breakage” is one potential adverse event. It also warns that “breakage of an embedded Paragard during non-surgical removal has been reported.” However, the documentation does not indicate how often such events are likely to occur.
Healthcare providers receive slightly more information about potential breakage issues. The removal instructions for healthcare providers warn that “the threads can retract into the uterus or break, or Paragard can break, perforate the uterus, or be expelled.” These instructions further warn healthcare providers that a broken or embedded Paragard IUD “can make removal difficult,” recommending various options to “assist in removing an embedded Paragard.”
Copper Toxicity
Copper is a naturally occurring element that is essential to human health in small amounts but can be toxic in excess. Copper is commonly used in IUDs because the metal is toxic to human sperm. In some cases, Paragard patients have reported symptoms consistent with copper toxicity.
Few medical studies currently suggest that Paragard can cause or contribute to copper toxicity. However, copper toxicity in association with the Paragard IUD has not been well-studied. A 2021 literature review found that 12 available studies examining blood copper levels and copper-containing IUD use were “inconclusive.” Additionally, eight studies found no connection between copper IUD use and increased blood copper levels. Meanwhile, four studies found increases in blood copper levels for copper IUD users. In addition, the researchers noted, all the studies measured total copper, not merely toxic “free copper…which raises questions about the clinical significance of all research on this subject to date.”
Copper toxicity may be a more complex question in the case of patients who have Wilson’s disease, a condition that impairs the body’s ability to get rid of excess copper. Symptoms are often neurological, psychological, or hepatological. They may include vomiting, muscle stiffness or weakness, fluid buildup in the abdomen, yellowish skin, tremors, trouble speaking, anxiety, or psychosis.
Paragard IUD Litigation Claims
To date, lawsuits involving the Paragard IUD focus on claims sounding in product liability, including manufacturing defects, design defects, and failure to warn.
Manufacturing & Design Defects
Both manufacturing and design defects claims focus on the Paragard IUD’s risks of breaking inside the uterus. This breakage may occur either during ordinary use or during the removal process. The argument is that a properly manufactured and designed IUD would not break during use or removal.
Specifically, some claims argue that the plastic used to make the upper arms of the IUD becomes hard and brittle over time. When a physician pulls on the IUD’s strings to remove the device, these arms break off. As a result, the pieces remain inside the uterus rather than folding upward as intended to allow the device to navigate the cervix. When the arms break, surgical intervention may be required for removal.
Failure to Warn
In addition, many claims argue that Teva Pharmaceuticals and Cooper Companies, manufacturers and distributors of the Paragard IUD, had a duty to warn consumers about the risk of breakage. According to users, these parties failed to meet their duty. Patients allege they were thus unaware of the risks of Paragard use, even if their doctors were informed.
Related to failure to warn claims are concerns about informed consent for Paragard insertion. As its name implies, “informed consent” requires that patients have sufficient information to make a thoughtful decision about their care. Patients who did not have access to information about the risks of Paragard breaking on removal may choose to argue, therefore, that their consent cannot have been informed.
Paragard IUD Recalls
Despite the wave of lawsuits linked to Paragard breakage or embedding, the FDA has not recalled the Paragard IUD from the market. Neither Cooper Companies nor Teva Pharmaceuticals have issued a voluntary recall of the device, either.
A medical device may be recalled in one of two ways. A voluntary recall is issued by the manufacturer or distributor of a product. This recall type is usually a means to protect public health after concerns about a device arise. Voluntary recalls are the most common type of recall.
In rare cases, the FDA issues a recall itself. Known as a mandatory recall, these recalls typically occur after a manufacturer or distributor has declined to issue a voluntary recall of a product shown to cause harm. To date, neither type of recall has implicated the Paragard IUD.
Expert insights have never been more important
Paragard Legal Proceedings
Currently, between 80 and 90 active cases name broken or embedded Paragard IUDs. This number is expected to rise, however, as patients become aware of their ability to participate in claims against Teva and Cooper.
Plaintiff Stephanie Ideus, along with others, filed a lawsuit against Teva Pharmaceuticals in 2016. According to the complaint, Ideus’s Paragard IUD broke and embedded in her uterus during removal. She required surgery to complete the removal.
In February 2019, the court granted Teva summary judgment in Ideus’s case. A three-judge panel of the Eighth Circuit upheld the ruling. The panel noted that Nebraska law did not require Teva to warn Ideus or other Paragard users directly about the risk of breakage. Under Nebraska’s learned intermediary doctrine, said the court, Teva only had a duty to warn Ideus’s physicians about the breakage risks. Per the court, the company met this duty.
On December 16, 2020, The Judicial Panel on Multidistrict Litigation (JPML) ordered that 55 Paragard IUD cases pending in 31 districts be consolidated in the US District Court for the Northern District of Georgia. There are currently 25 related cases that may also be included in the MDL.
In its ruling, the JPML rejected Teva and Cooper’s claims that variations between plaintiffs in these cases made them unsuitable for consolidation. The panel also rejected the defendants’ arguments that the cases should not be consolidated because they would fail as a matter of law. Here, the JPML noted it has no authority to consider the action’s merits as part of its consolidation decision.
The JPML found that the Paragard cases “involve common questions of fact,” including allegations that Paragard “has a propensity to break upon removal, causing complications and injuries, including surgeries to remove the broken pieces of the device, infertility, and pain. The actions thus implicate questions concerning the device’s development, manufacture, testing, labeling, and marketing.”
Challenges to Proving a Paragard IUD Case
A few challenges arise in proving a product liability case involving a Paragard IUD. These include demonstrating that the IUD’s makers and distributors had a duty to warn patients directly, demonstrating manufacturing or design defects, and drawing causal connections between IUD use and medical conditions like uterine scarring or copper toxicity.
As Ideus’s case demonstrates, establishing a failure to warn claim can be a challenge. The Eighth Circuit panel held that Nebraska law did not require Teva Pharmaceuticals to warn patients about the risk of breakage, only to warn physicians. Where the “learned intermediary” doctrine applies, it is likely to pose an obstacle to attorneys seeking to establish defendants’ responsibility to warn Paragard users that breakage might occur.
Similarly, attorneys may need to establish that manufacturing or design defects exist and cause problems like device breakage or copper toxicity. Causation poses a number of challenges, particularly since many symptoms associated with Paragard use are also common menstrual experiences. Meanwhile, research on copper toxicity associated with Paragard use remains inconclusive.
Expert Witnesses and Paragard IUD Litigation
Expert witness testimony will be essential in Paragard IUD manufacturing defect, design defect, and failure to warn claims. Depending on the specific claims asserted, various experts and their testimony may be required.
Medical device manufacturing specialists will be called on for questions surrounding the fabrication, design, and basic function of the Paragard IUD. Additionally, these experts may opine on the factors that can cause the device to break. Similarly, design experts will play an essential role on the legal team. These experts can examine examples of broken or whole Paragard IUDs and opine on how breakage occurred.
To establish causation and damages, medical experts may be required. Gynecology experts, in particular, can teach the fact-finders key information about the anatomy of the uterus. In addition, these experts may opine on the connection between a broken or embedded Paragard IUD and the need for surgical removal of IUD pieces. Their testimony may also include information on medical conditions like uterine scarring, infertility, or copper toxicity.
Additional medical experts may be needed in cases where a patient appears to have suffered copper toxicity. A hepatologist, for example, may be able to formulate and share an expert opinion on the patient’s liver health. A hepatology expert can also explain the liver’s connection to the ability of the patient’s body to expel excess copper effectively.
Additionally, a toxicologist can explore the effects of excess copper on the body and how these may relate to Paragard use. A toxicology expert can also opine on what symptoms are likely to occur when the levels of free copper in the bloodstream rise to toxic levels.
Digging into the Paragard IUD Research
Like many cases involving defective medical devices, the claims surrounding Paragard IUD use can be complex. For clarity, attorneys can turn to the Expert Institute’s new Strategic Research service.
When an attorney requests a report from the Strategic Research service, our team of in-house physicians will compile and analyze all the relevant industry research. This includes:
- Reviewing the available scientific and peer-reviewed literature
- Distilling this information into an easy-to-read guide
- Outlining potential causation arguments
- Exploring preexisting conditions that may complicate the question of causation
- Offering recommendations on how to pursue a winning case
When it comes to a Paragard IUD case, our physicians are your on-call experts for understanding the full breadth of Paragard IUD side effects, breakage, copper toxicity, and related concerns.
This is all delivered via an easy-to-digest guide—jumpstarting your Paragard IUD case preparations. Save your team from hours of research legwork and request your first Guidebook today.
About the author
Wendy Ketner, M.D.
Dr. Wendy Ketner is a distinguished medical professional with a comprehensive background in surgery and medical research. Currently serving as the Senior Vice President of Medical Affairs at the Expert Institute, she plays a pivotal role in overseeing the organization's most important client relationships. Dr. Ketner's extensive surgical training was completed at Mount Sinai Beth Israel, where she gained hands-on experience in various general surgery procedures, including hernia repairs, cholecystectomies, appendectomies, mastectomies for breast cancer, breast reconstruction, surgical oncology, vascular surgery, and colorectal surgery. She also provided care in the surgical intensive care unit.
Her research interests have focused on post-mastectomy reconstruction and the surgical treatment of gastric cancer, including co-authoring a textbook chapter on the subject. Additionally, she has contributed to research on the percutaneous delivery of stem cells following myocardial infarction.
Dr. Ketner's educational background includes a Bachelor's degree from Yale University in Latin American Studies and a Doctor of Medicine (M.D.) from SUNY Downstate College of Medicine. Moreover, she is a member of the Board of Advisors for Opollo Technologies, a fintech healthcare AI company, contributing her medical expertise to enhance healthcare technology solutions. Her role at Expert Institute involves leveraging her medical knowledge to provide insights into legal cases, underscoring her unique blend of medical and legal acumen.
Subscribe to our newsletter
Join our newsletter to stay up to date on legal news, insights and product updates from Expert Institute.
Working on a Paragard IUD case?
We're here to help you build a stronger case. Retain a leading expert witness today.
- Access tailored expertise on every case
- Trust your expert immediately
- Speak with your expert before retaining
Need your medical records reviewed? Consult one of our 75+ on-staff physicians who can help evaluate the strengths and weaknesses of your case.




